Near-Infrared spectroscopy (NIRS)
Near-Infrared Spectroscopy (NIRS) measures light attenuation due to absorption of hemoglobin, and from the absorption at two or more wavelengths, it estimates changes in hemoglobin concentration (HbT, or cerebral blood volume (CBV)) and quantifies cerebral hemoglobin oxygenation (SO2).
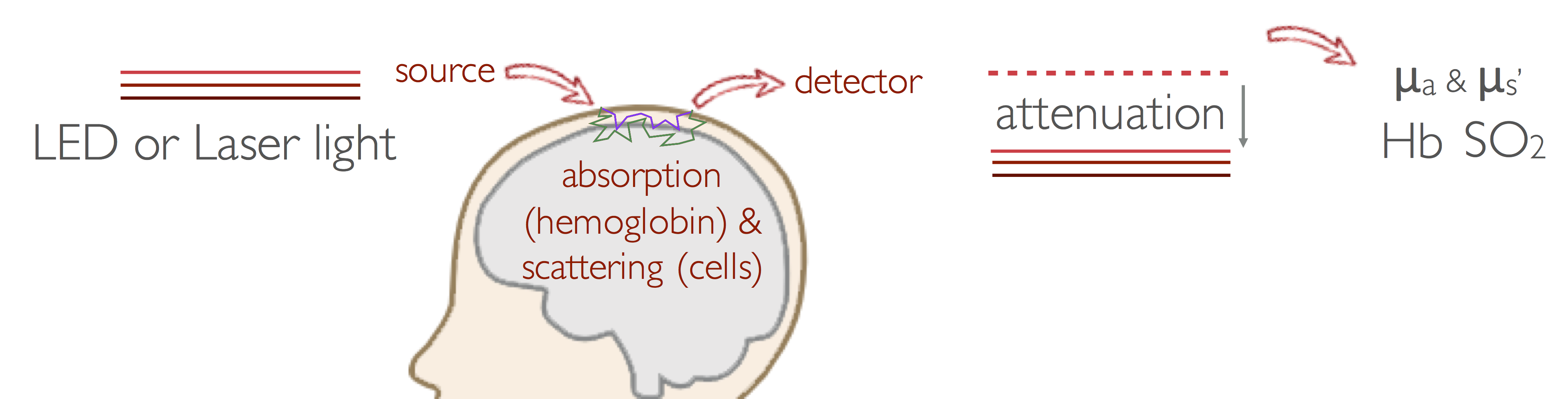
Our group led the development of one of the first imaging systems using continuous-wave near-infrared spectroscopy (CW-NIRS) for functional brain imaging, which is being disseminated commercially by Techen, with customers in North America, Europe, Asia, Australia, and Brazil. We have also contributed to the development of frequency-domain (FD) and time-domain (TD) NIRS systems to improve quantification of optical properties of tissue and to increase sensitivity to deeper structures. We are now working on the miniaturization of these systems, and development of wearable wireless devices.
Diffuse Correlation Spectroscopy (DCS)
DCS was developed in the ’90s by Dr. David Boas and Dr. Arjun Yodh. Since then, DCS has been widely adopted and its utility is now being tested in several clinical applications. DCS measures how fast coherent light loses coherence because of the movement of red blood cells. The correlation diffusion equation relates the motion of red blood cells in vessels to the temporal autocorrelation decay. Since the correlation decay depends on both the speed of moving red blood cells in the media and on the number of scattering events with the moving particles, which depends on the area of the blood vessels, the slope of the correlation decay is proportional to actual blood flow and not simply flow velocity as in ultrasound methods. By fitting the correlation diffusion equation to the measured autocorrelation we derive a cerebral blood flow index (CBFi, cm2∕s).
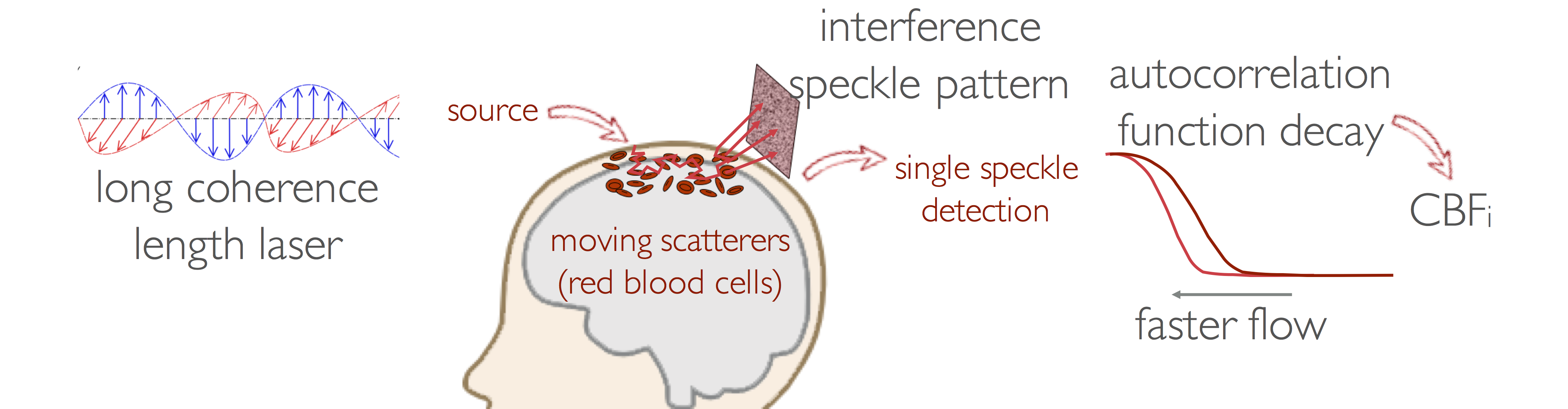
Our group started adapting DCS for clinical studies in 2006. Since then, we have made several advances in DCS theory and instrumentation, which we have applied in animals4 and infants to establish CBFi normative values and demonstrate differences with therapy and disease. We have also used DCS as a continuous monitor, first demonstrating the ability of DCS to measure CBFi changes during functional activity in infants, then moving into clinical applications as a powerful neuromonitoring tool.
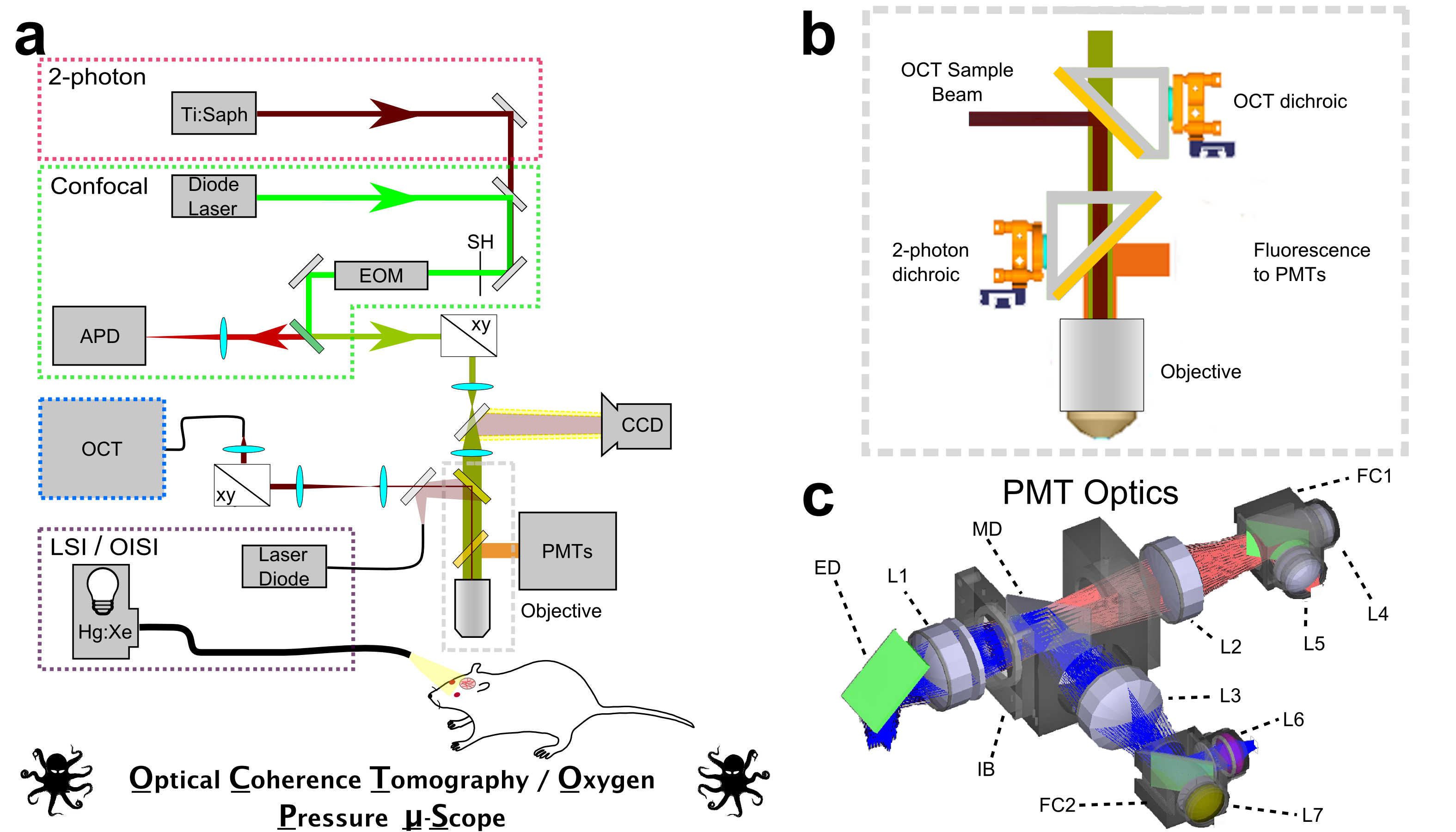
Optical Coherence Tomography (OCT)
We are developing optical coherence tomography methods to measure microvascular flow characteristics, intracellular organelle motility, and neuronal activity. We have been developing Optical Coherence Tomography methods for in vivo imaging of vascular and cellular dynamics in living animal brains. We are developing novel methods that in addition to providing quantitative measurements of cerebral blood flow, permit quantification of capillary red blood cell flux and speed over hundreds of capillaries with seconds temporal resolution. Combining OCT with dynamic light scattering, we are demonstrating the ability to quantify the diffusion of intracellular organelles in neuronal cell bodies with micrometer resolution, hundreds of micrometers deep in the in vivo rodent brain. These were measurements that were only previously possible in cell cultures and open the possibility of exploring cellular vitality in vivo in response to pathological challenges.
Laser-Scanning Microscopy
We devote considerable effort to developing microscopy-based methods to noninvasively measure hallmarks of cerebral energy metabolism and blood flow in living preclinical models. We have developed confocal- and multiphoton- lifetime microscopy techniques for measuring cerebral oxygenation using meticulously designed, oxygen-sensitive phosphorescent contrast agents. Our group reported the first ever noninvasive, high-resolution observations of oxygen partial pressure in living brain tissue, providing novel insight into cerebral oxygen delivery and consumption at the microscopic length scale.
We have also developed and applied microscopy techniques to monitor fluorescence lifetime of intrinsic NADH as a marker of mitochondrial energy metabolism. NADH is ubiquitous within cells and plays multiple roles in anaerobic and aerobic energy metabolism. We apply fluorescence lifetime measurements of NADH to observe subtle variations in mitochondrial activity in the living cortex of preclinical models.
Observing these facets of cerebral energetics and blood flow at the microscopic scale within the intact brain is crucial to guide our understanding of brain function and its pathological alterations. These investigations will help identify the cellular and physiological underpinnings of observations collected with more translatable technologies such as Near Infrared Spectroscopy and funcitonal Magnetic Resonance Imaging.
Multispectral Intrinsic Signal Imaging
We make extensive use of systems that light up brain tissue at different wavelengths. Essentially, oxy- and deoxy hemoglobin have different colors, indicative of their different absorption spectra. By shining light of different wavelengths onto the exposed skull or brain surface and recording images with a camera, we can obtain detailed information about regional and temporal changes in blood oxygenation.
Laser Speckle Contrast Imaging (LSCI)
Several years ago our group introduced Laser Speckle Contrast Imaging (LSCI) to the neurosciences making it possible for the first time to obtain images of cerebral blood flow with high spatial and temporal resolution.
